Message

Toshiaki Takagi
Chairman
Medical Technology Association of Japan
With the fight against COVID-19 apparently working out for the better, many of us have begun to return to our daily lives. I would like to express my sincere gratitude to all the healthcare professionals who have devoted themselves to patients for more than three years and to all the member companies who have made every effort to ensure a stable supply of medical devices under the slogan “Never disrupt healthcare.”
In 2013, the former Japan Medical Devices Manufacturers Association (JMED) was renamed to the Medical Technology Association of Japan (MTJAPAN). At that time, the products we handled were fast expanding to include regenerative medicine, ICT, software, systems, and many other fields, so we made a new start aiming at promoting medical technology beyond conventional medical devices and materials. Over the past ten years, the environment surrounding us has changed even more dramatically.
In the face of COVID-19, the wave of digital transformation (DX) is sweeping even in the world of healthcare, which has been said to be lagging behind in the use of digital technology. It is estimated that there will be a shortage of nearly 1 million healthcare professionals by 2040, so there is no time to waste in rethinking the way we work and improving efficiency through digitalization. The medical devices handled by MTJAPAN also require unprecedented ideas not only for therapeutic effects but also for improving the efficiency and eliminating healthcare disparity. We will actively collaborate with companies in different fields and industries and work even harder to meet the challenge of new technologies.
There is no stopping globalization. As the medical device market in the world, excluding Japan, continues to grow, the overseas expansion of member companies is accelerating, with the ratio of their overseas sales increasing significantly over the past decade. Despite some protectionist movements there, globalization cannot be reversed anymore. We would like to continue to work with the government to further support globalization, for example, by promoting the coordination of regulations that hinder overseas expansion.
From the viewpoint of ensuring the security of healthcare, the medical device industry is facing ever-greater responsibilities and expectations. We will contribute to improving the quality and efficiency of healthcare around the world by developing excellent medical devices while at the same time fulfilling our social responsibility to ensure a stable supply of such devices.
No matter how much the environment changes, we must not forget to ensure thorough compliance. Medical device manufacturers are required to have high ethical standards. We will work even harder to ensure compliance at all levels, keeping in mind that compliance is not only about complying with laws and regulations but also about adhering to social norms and ethical standards.
The chaotic global environment is likely to keep us in a challenging business situation for some time to come, and I would like to ask all of MTJAPAN’s stakeholders to continue to understand and cooperate with us.
Overview
Promptly providing safe and innovative medical technology Contributing to improved medical quality and the development of the medical device technology industry
Summary
Medical Technology Association of Japan
【Regular members】 266 companies
【Associate members】 40 companies
【Number of employees】
Japan:Approx. 83,000
Outside Japan: Approx. 109,000
As of June 2023
History
- 1967:
- Medical Plastics Conference established. 1990:Name changed to Japan Association of Medical and Material Industries (JAMMI)
- 1979:
- Japan Artificial Organ Industry Association (JAOIA) established
- 2000:
- JAMMI and JAOIA merged. Japan Medical Devices Manufactures Association (JMED) launched
- 2013:
- Reorganized as Medical Technology Association of Japan (MTJAPAN)
- 2023:
- 10th anniversary of Medical Technology Association of Japan (MTJAPAN) Establishment
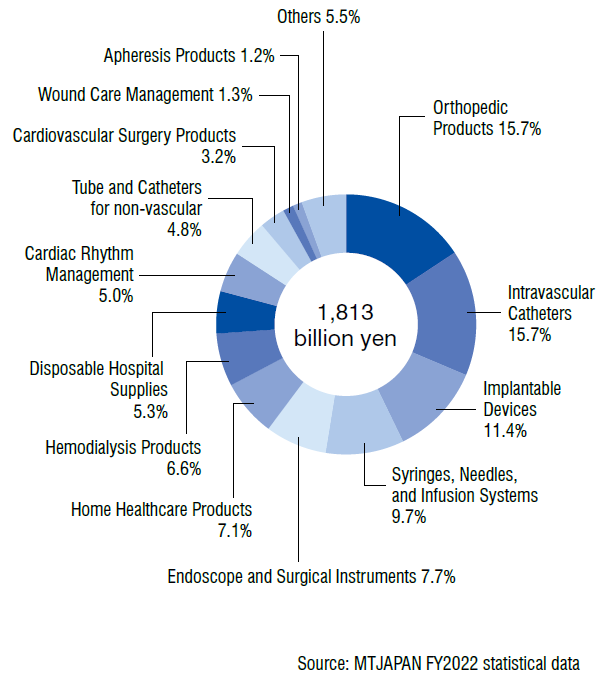
Composition of Domestic Shipment for MTJAPAN products
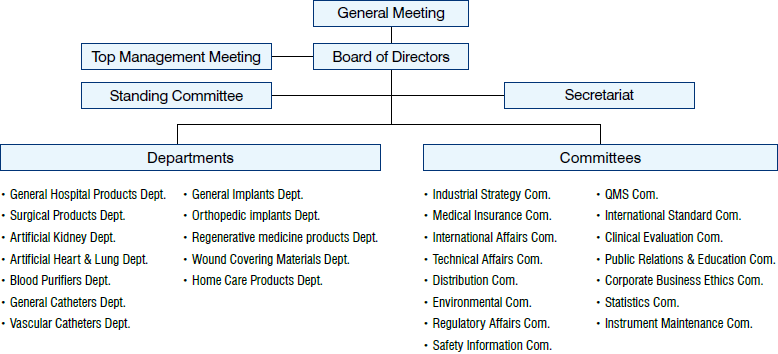
Organization
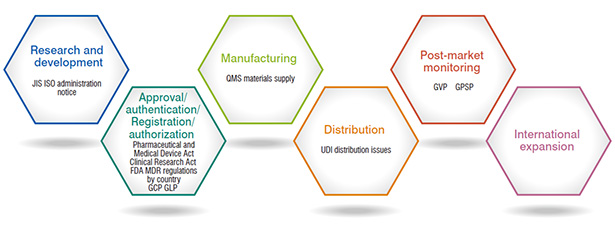
Activity
Development of business from R&D to post-commercialization and international expansion
- To maintain the effectiveness and safety of medical devices
- Maintaining stable supply
Business for member companies
- New administration and industry trends declarations
- Hosting of training seminars and lectures, etc., for members only
- Presenting opportunities to configure networks with the industry and government, etc.
- Proposing corporate requirements to government as a voice for the industry
- Other businesses for members
Publishing MTJAPAN news, and compiling MTJAPAN medical device statistical data Operating medical technology matching sites and seminars Introducing MTJAPAN group insurance (product liability insurance both in Japan and other countries) Assuring stable supplies during a disaster
Announcing government and industry trends
We periodically distribute notices from the government and offices on the MTJAPAN members website.
Website top page
Lectures and seminars
We organize lectures and seminars on the latest regulatory trends and business and employee training for companies.
- Lecture on medical insurance system
- Lecture on medical device regulations
- Lecture on clinical evaluation system
- Lecture on QMS compliance survey
- Lecture on trends in international standards
- Medical device technology matching seminar
- Overseas business expansion seminar
- Medical device distribution seminar
- Environmental regulation seminar
- Public relations seminar
- Post market surveillance workshop
- Corporate ethics education workshop
- Continuous training for selling and repairing medical devices*
(*Held in the capacity of a Ministry of Health, Labour and Welfare registered organization)

Continuous sales and repair training

Public relations seminar

General meeting
Domestic and foreign networking
We provide networking opportunities with the medical device industry and government through employee general meetings/gatherings, New Year's gatherings, committees, and group activities.
We create global networks. Our activities include sending representatives to international conferences like AdvaMed, APACMED and AHWP, as well as hosting medical device symposiums and booth exhibitions at CHINA-HOSPEQ.

MTJAPAN exhibition booth
Announcing policy proposals
Through committee activities, we announce policy proposals following discussions and meetings with the Japan Federation of Medical Devices Associations (JFMDA) and the government regarding policies concerning industrial development, health insurance systems, laws and regulations, and standards.

Gathering details
Participation in setting ISO and JIS standards
As the Japanese consultative body for the technical committee of four ISO fields, we send representatives to international conferences and participate in setting international standards.
- ISO/TC76 (Blood transfusion and infusion equipment, etc.)
- ISO/TC84 (Injection equipment, etc.)
- ISO/TC150 (Artificial lungs, surgical implant devices, etc.)
- ISO/TC194 (Biological safety evaluations for medical devices)
We have participated in establishing approx. 80 JIS standards concerning our organization's products.

ISO/TC84 Tokyo Conference
Publishing printed materials
We edit and publish materials concerning medical devices.We also introduce activities statuses and data through our website.
- Special Insurance Medical Materials Guidebook
Posting use objectives, methods, fees, and redemption prices, etc., for special insurance medical materials - Introduction to plastic medical devices
Posting plastic materials and characteristics for sterilization devices - MTJAPAN medical devices statistical data
Posting market and industry trends within the scope of our organization - MTJAPAN news (website)
Introducing activities statuses for our organization - Medical device past and present stories (website)
Introducing the history of various medical devices

Special Insurance Medical Materials Guidebook

MTJAPAN medical devices
statistical data
Matching site operations
We operate a matching site on our website as a forum for meeting MTJAPAN member companies, companies in different fields, the academic society and other organizations. Member companies can browse information including products and technologies registered by companies in other fields and use the information to introduce new technologies needed to differentiate their products. We offer opportunities for companies in other fields to enter the medical device industry.
https:www.mtjapan.or.jp/jp/matching/
Matching website
